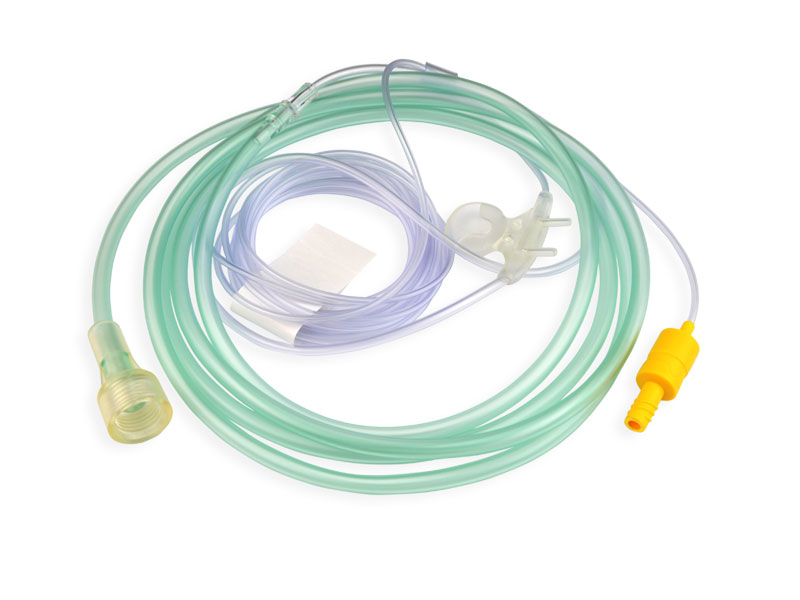
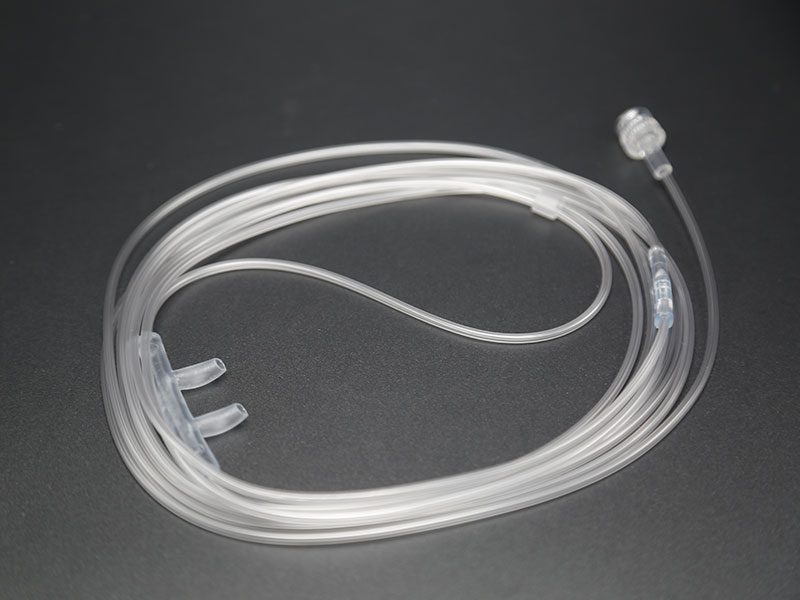
Disposable non-invasive EEG sensor, also known as anesthesia depth EEG sensor. It is mainly composed of electrode sheet, wire and connector. It is used in combination with EEG monitoring equipment to noninvasively measure patients' EEG signals, monitor the anesthesia depth value in real time, comprehensively reflect the changes of anesthesia depth during operation, verify the clinical anesthesia treatment scheme, avoid the occurrence of anesthesia medical accidents, and provide accurate guidance for intraoperative awakening.

The disposable non-invasive EEG sensor independently developed and designed by Medlinket medical has passed the registration and certification of the China National Medical Products Administration (NMPA) since 2014 and has been recognized for renewal for many years. Therefore, it has also been favored by hundreds of well-known hospitals in China. Many hospitals have chosen Medlinket disposable non-invasive EEG sensors for many years to be used in operating rooms, anesthesiology departments, ICU and other departments, which is also the recognition and trust of Medlinket disposable non-invasive EEG sensors.
After years of clinical verification, Medlinket has developed various EEG sensors compatible with anesthesia depth technology, including dual channel EEG dual frequency index anesthesia depth EEG sensors for adults and children; Entropy index EEG sensor; EEG state index sensor; There are four channel EEG dual frequency index sensors; There are also the newly developed IOC anesthesia depth EEG sensor and various adapters connected to the EEG sensor. At present, the types of Medlinket EEG sensors basically cover most of the EEG sensors needed in clinic.
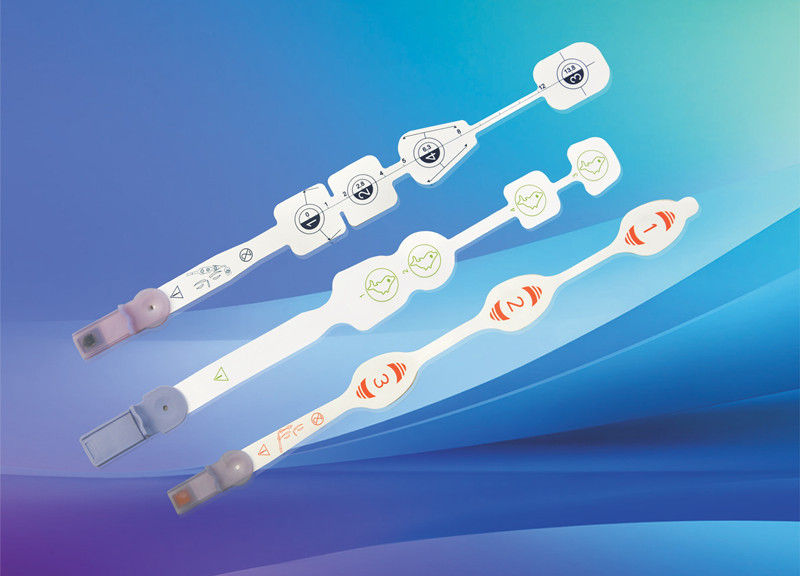
In addition to its clinical application in domestic hospitals, Medlinket has also passed the CE certification and entered the EU market. The U.S. market is being submitted for inspection. It is believed that it will soon pass the registration and approval of the U.S. FDA and enter the American market to help the in-depth monitoring of anesthesia in medical surgery at home and abroad.
Statement: All the above content shows the registered trademark, name, model, etc., the ownership of the original holder or the original manufacturer, this article is only used to illustrate the compatibility of the United States even products, nothing else! All the above information is for reference only, do not use as medical institutions or related units work guide, otherwise, cause any consequences and the company has nothing to do.
Previous: Why choose a disposable non-invasive EEG sensor adapted to BIS module?
Next: Medlinket depth-of-anesthesia sensor helps anesthesiologists for difficult surgeries!
Related News
- Why does anesthesiology department use disposable spo2 sen...
- Medlinket's physical sign monitoring equipment is a "good...
- Medlinket won the Top 10 Best Reputation Equipment a...
- Medlinket's Infant Incubator, Warmer Temperature Probe...
- Long-term SpO2 monitoring will cause skin burn risk?
- New product recommendations:Medlinket disposable IBP ...
- Medlinket's NIBP cuff adapts to the needs of different d...
- Medlinket's Compatible Welch Allyn Smart Temp Prob...
- How to choose a reusable SpO2 sensor?
- Medlinket disposable EEG sensors to provide accurate mo...
- Medlinket's pelvic floor muscle rehabilitation probe helps...
- Pelvic floor rehabilitation probe manufacturer, manufactu...
- Why is the body cavity temperature probe generally selecte...
- Medlinket's One-Piece ECG Cable with LeadWires is ...
- Medlinket's blood oxygen probe is highly accurate, escortin...
- Medlinket professionally developed a high-precision oximet...
- Medlinket's products obtaining the UK MHRA registrat...
- Medlinket's disposable NIBP cuff protector can effective...
- Medlinket disposable defibrillation electrode are registere...
- Medlinket's Y-type multi-site SpO2 probe, a small expe...
- Medlinket’s disposable NIBP cuff, specially designed fo...
- Medlinket's disposable non-invasive EEG sensor helps to ...
- Pelvic floor rehabilitation probe manufacturer, recognize ...
- Pelvic floor rehabilitation can not be ignored ,Look for M...
- Medlinket Digital Infrared Thermometer, a good helper f...
- End expiratory carbon dioxide sensor and sampling tube acc...
- Medlinket's temp-pulse oximeter realizes five major heal...
- Low SpO2, have you found the reason behind it?
- Manufacturers of disposable EEG sensors prefer Medlinke...
- Medlinket’s home portable Temp-Pluse oximeter, scient...
- Why does anesthesiology department use disposable spo2 sen...
- Medlinket's disposable temperature probe meets the needs ...
- Medlinket Temp-plus oximeter, the patron saint of health...
- How to choose suitable disposable SpO2 sensor in differe...
- Medlinket anesthesia depth EEG sensor has obtained MHR...
- Medlinket's disposable NIBP cuff can effectively reduce ...
- The manufacturer of pelvic floor rehabilitation probe is t...
- NIBP measurement method and choice of NIBPcuff
- Medlinket's internal electrode for pelvic floor muscle the...
- Expert consensus on emergency end expiratory carbon dioxid...
- What types of oximeters are there? How to buy it?
- For ETCO2 monitoring, intubated patients are most sui...
- Medlinket's anti-jitter high-precision Temp-Pluse oxime...
- A high-precision oximeter that meets clinical testing, a
- Disposable non-invasive EEG sensor, supplied by the man...
- What are the characteristics of Medlinket’s new silicone...
- In 2021 CMEF/ICMD autumn exhibition, Medlinket invi...
- How to select spo2 sensor in various departments of the h...
- How is Medlinket's disposable non-invasive EEG sensor ...
- Internationally acclaimed oximeter——Medlinket’s tempe...
- What are the types of disposable non-invasive EEG sensor...
- To monitor the patient's respiratory status, it is necessa...
- The clinical significance of temperature management duri...
- The difference between disposable Skin-surface temperatu...
- Why should we use disposable noninvasive EEG sensors to...
- "Guardian God" for Premature Infants-ncubator Temperat...
- What is the difference between mainstream CO2 sensor a...
- The importance of disposable temperature probes in clinic...
- Application scenarios and usage methods of Disposable Sp...
- For the bidding of disposable EEG sensor manufacturers,...
- Will the SpO2 sensor cause neonatal skin burns in SpO2...
- How to choose the appropriate disposable anesthesia depth ...
- SpO2 of Novel Coronavirus Pneumonia testing standards
- Application scenarios and applicable people of different S...
- Medlinket's ETCO2 mainstream and sidestream sensors ...
- Why choose a disposable non-invasive EEG sensor adapted ...
- Medlinket's disposable non-invasive EEG sensor has been ...
- Medlinket depth-of-anesthesia sensor helps anesthesiologist...
- Medlinket Adult Finger Clip Oximetry Probe, a great h...
- FIME2018
- 2018 Med-link Medical Exhibitions Preview
- [Exhibition notice] Med-linket’s exhibition overview in ...
- 2017 American Society Of Anesthesiologists Annual Con...
- [Exhibition Invitation]Med-linket Invited You To Partic...
- Med-link Participated In The 2017 Annual Meeting Of A...
Related Products









 marketing@medxing.com
marketing@medxing.com



